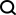Home >Technology peripherals >AI >SOTA performance, Xiamen multi-modal protein-ligand affinity prediction AI method, combines molecular surface information for the first time
SOTA performance, Xiamen multi-modal protein-ligand affinity prediction AI method, combines molecular surface information for the first time
- WBOYWBOYWBOYWBOYWBOYWBOYWBOYWBOYWBOYWBOYWBOYWBOYWBOriginal
- 2024-07-17 18:37:101467browse

Editor | KX
In the field of drug research and development, accurately and effectively predicting the binding affinity of proteins and ligands is crucial for drug screening and optimization. However, current studies do not take into account the important role of molecular surface information in protein-ligand interactions.
Based on this, researchers from Xiamen University proposed a novel multi-modal feature extraction (MFE) framework, which for the first time combines information on protein surface, 3D structure and sequence, and uses a cross-attention mechanism for different modes. Feature alignment between states.
Experimental results show that this method achieves state-of-the-art performance in predicting protein-ligand binding affinity. Furthermore, ablation studies demonstrate the effectiveness and necessity of protein surface information and multimodal feature alignment within this framework.
Related research titled "Surface-based multimodal protein–ligand binding affinity prediction" was published on "Bioinformatics" on June 21.

Protein-ligand binding affinity prediction research
As a key stage of drug discovery, predicting protein-ligand binding affinity has been extensively studied for a long time, which is crucial for efficient and accurate drug screening.
Traditional computer-aided drug discovery tools use scoring functions (SF) to roughly estimate protein-ligand binding affinity, but with low accuracy. Molecular dynamics simulation methods can provide more accurate binding affinity estimates but are often costly and time-consuming.
With the development of computing technology and the increasing abundance of large-scale biological data, deep learning-based methods have shown great potential in the field of protein-ligand binding affinity prediction.
However, current research mainly utilizes sequence- or structure-based representations to predict protein-ligand binding affinity, and there are relatively few studies on protein surface information that is crucial for protein-ligand interactions.
A molecular surface is a high-level representation of a protein's structure, which exhibits characteristic chemical and geometric patterns that serve as fingerprints of the protein's interaction patterns with other biomolecules. Therefore, some studies began to use protein surface information to predict protein-ligand binding affinity.
But existing methods mainly focus on single-modal data and ignore the multi-modal information of proteins. Furthermore, when processing multimodal information of proteins, traditional methods usually connect features from different modalities in a direct manner without considering the heterogeneity between them, which results in the inability to effectively exploit the complementarity between modalities. .
Novel multi-modal feature extraction framework
Here, researchers propose a novel multi-modal feature extraction (MFE) framework that for the first time combines information from protein surface, 3D structure and sequence .
Specifically, the study designed two main components: protein feature extraction module and multi-modal feature comparison module.
The protein feature extraction module is used to extract initial embeddings from protein surface, structure and sequence information.
In the multi-modal feature comparison module, the cross-attention mechanism is used to achieve feature comparison between protein structure, sequence embedding and surface embedding to obtain a unified and information-rich feature embedding.
Compared with current state-of-the-art methods, the proposed framework achieves the best results on the protein-ligand binding affinity prediction task.
SOTA Performance
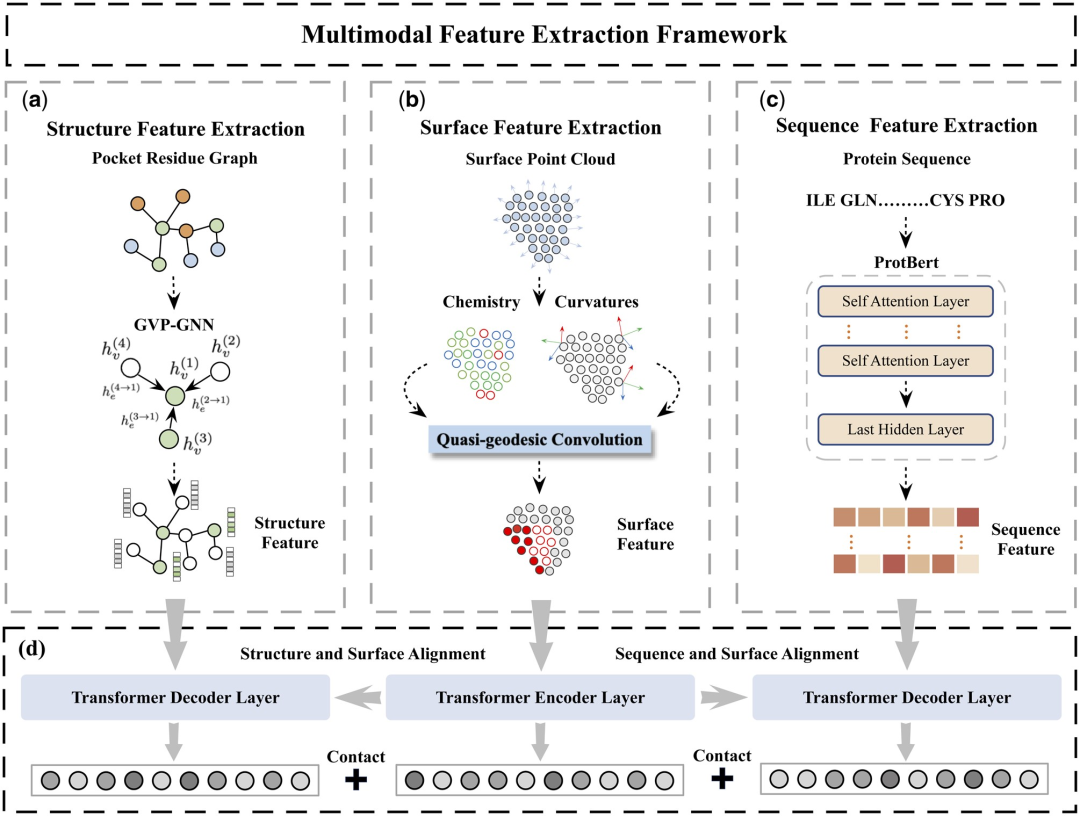
Table 1 shows the results of MFE and other baseline models on the protein-ligand binding affinity prediction task. All models used the same training and validation set partitioning method and were tested on the PDBbind core set (version 2016). It can be found that the MFE method achieves SOTA performance compared to all baselines.
Ablation Study
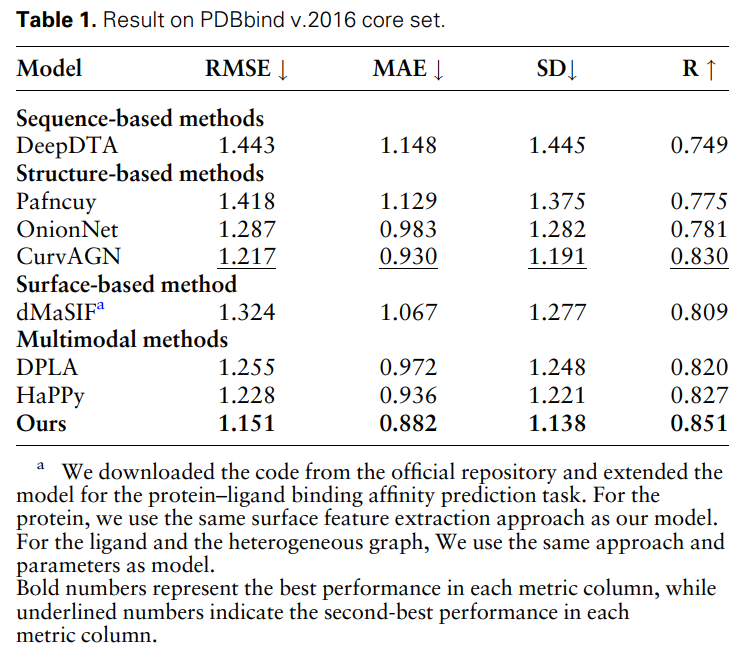
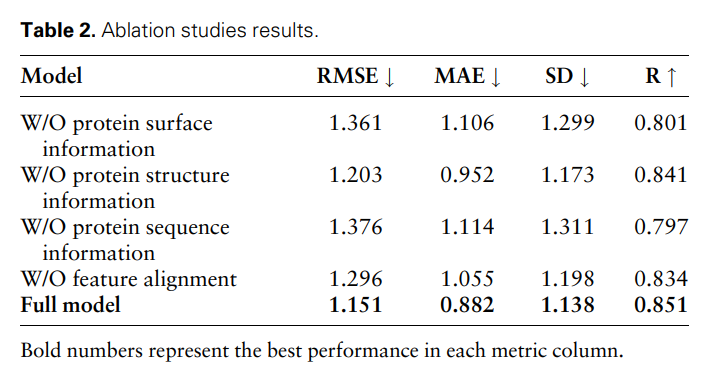
To further prove the effectiveness and necessity of different modal features and feature comparisons, the researchers conducted the following ablation studies: W/O protein surface information, W/O protein structure information , w/o protein sequence information and featureless alignments. The results are shown in Table 2 and Figure 2.
Figure 2: Ablation study results. (Source: paper)
The results show that when surface information is removed, the performance drops significantly, indicating that surface information plays a crucial role in the model. Likewise, excluding either structural or sequence information results in performance degradation, while elimination of sequence information results in a more pronounced degradation. This is because sequence information contains global information about the protein, which is crucial for the model to fully understand the protein.
In addition, without feature comparison, the performance of the model will decrease. This emphasizes the importance of feature comparison in processing multi-modal data, as it helps reduce the heterogeneity between different modal features, thereby improving the model's ability to effectively integrate different modal features.
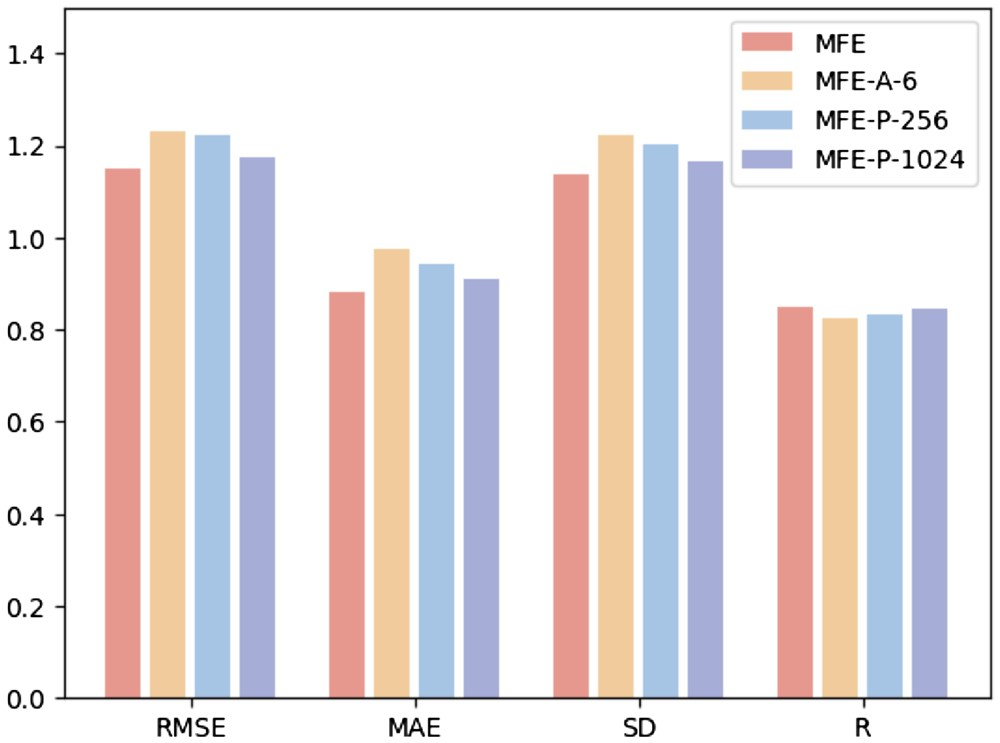
Hyperparameter analysis
In order to study the impact of different hyperparameters on model performance, the researchers conducted the following three experiments: (i) MFE-A-6: only use 6 basic atom types to represent Chemical properties of the surface, including hydrogen, carbon, nitrogen, oxygen, phosphorus, and sulfur; (ii) MFE-P-256: Only the 256 surface points closest to the ligand center are selected as the protein pocket surface; (iii) MFE-P -1024: Select the 1024 surface points closest to the ligand center as the protein pocket surface.
Figure 3 shows the results of three different hyperparameter selection methods on the protein-ligand binding affinity prediction task.
Feature Alignment Analysis and Visualization
In order to deeply study the impact of feature alignment on model performance, the researchers used principal component analysis (PCA) to perform dimensionality reduction and summation of protein surface, structural and sequence features in the test set Visual analysis. This approach aims to determine whether feature alignment can mitigate heterogeneity between multimodal embeddings.
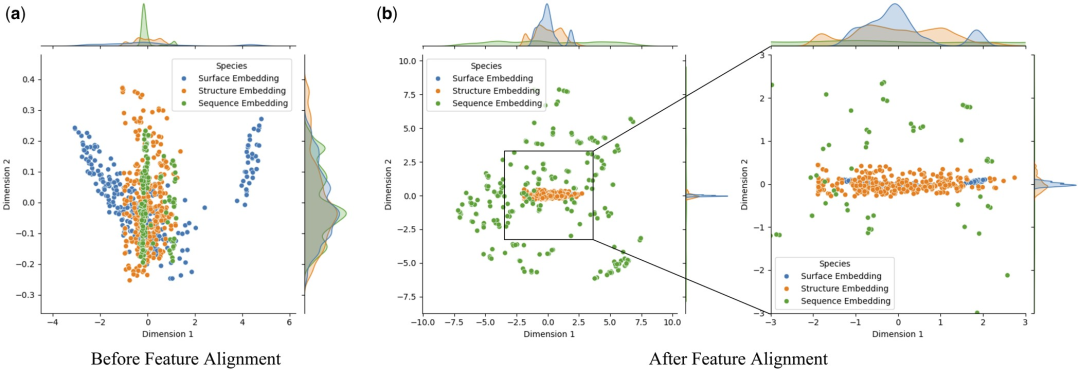
Research found that feature alignment significantly enhanced the consistency between protein surface, structure and sequence embedding. This is due to the optimization of multi-modal feature interactions in Transformer through the attention mechanism, which calculates attention weights between different features. This enhances the model's ability to capture key information, allowing data from different modalities to be more closely clustered in feature space, thereby reducing noise and errors in the model's identification of protein-ligand interactions.
Finally, the researchers concluded, “In summary, by studying the surface of proteins, we can gain a deeper understanding of how proteins interact with other biomolecules. In future work, we will explore protein surfaces more thoroughly to reveal Their wider application in bioinformatics"
Note: The cover comes from the Internet
.The above is the detailed content of SOTA performance, Xiamen multi-modal protein-ligand affinity prediction AI method, combines molecular surface information for the first time. For more information, please follow other related articles on the PHP Chinese website!
Related articles
See more- Technology trends to watch in 2023
- How Artificial Intelligence is Bringing New Everyday Work to Data Center Teams
- Can artificial intelligence or automation solve the problem of low energy efficiency in buildings?
- OpenAI co-founder interviewed by Huang Renxun: GPT-4's reasoning capabilities have not yet reached expectations
- Microsoft's Bing surpasses Google in search traffic thanks to OpenAI technology