 Technology peripherals
Technology peripherals AI
AI AI designs protein 'switches' from scratch, an amazing breakthrough in protein design, David Baker's research is published in Nature
AI designs protein 'switches' from scratch, an amazing breakthrough in protein design, David Baker's research is published in NatureAI designs protein 'switches' from scratch, an amazing breakthrough in protein design, David Baker's research is published in Nature

In life, it’s easy to turn on a light or adjust the light. But systems that achieve similar control of biomolecular functions are complex and poorly understood.
In biology, protein functions are turned on and off in complex ways. Allosteric regulation is one of the important biological regulatory mechanisms and is crucial for healthy metabolism and cell signaling. But creating allostery in synthetic protein systems has always presented significant challenges.
Recently, David Baker’s team at the University of Washington designed a protein that can switch between assembly and disassembly reliably and accurately through allosteric control. Using AI to design new proteins that do not exist in nature, researchers have engineered multiple dynamic protein arrangements.
David Baker said: "By designing proteins that can be assembled and disassembled on command, we are paving the way for future biotechnologies that may rival the complexity of nature."
# 🎜🎜#Arvind Pillai, first author and corresponding author of the paper, said: "One of the key innovations of this study is the design of protein assemblies that can switch between different oligomer states, such as dimers. , rings and cages in response to effector molecules, this ability to remotely control protein structure opens up the possibility of developing adaptive biomaterials and drug delivery systems. A stunning breakthrough in design." The relevant research was titled "De novo design of allosterically switchable protein assemblies" and was published in "Nature" on August 14.Allostery and de novo design
 Paper link: https://www.nature.com/articles/s41586-024-07813-2#🎜 🎜#
Paper link: https://www.nature.com/articles/s41586-024-07813-2#🎜 🎜#
- Proteins designed to change structure and function in response to specific molecular signals are called
- Allosteric Regulation#🎜🎜 #.
De novo design
De novo designed proteins expand the repertoire of naturally evolved properties, opening the door to more controllable control of protein function.- Inspiration: Monod-Wyman-Changeux (MWC) collaborative model
- allosteric
- .
Design strategy
- Use the MWC model as a starting point for design
- Utilize protein structure prediction tools# 🎜🎜#
- Structural Switch
- Sewing protein modules in a structurally and energetically feasible way
- #🎜 🎜#Illustration: Design strategies for building switchable oligomers. (Source: paper)
-
Researchers demonstrated the application of RFdiffusion, ProteinMPNN and other design tools, For creating a series of dynamic and conformational switching protein assemblies. By combining two-state hinges and customized protein-protein interaction modules, the resulting assemblies are significantly different from any previously seen, expanding the possibilities of synthetic biology. Research results:
Research results:
Key Innovation:
A key innovation in this research is the design of protein assemblies. In addition to structural versatility, the team also achieved high-affinity binding between the new protein and its effectors, ensuring reliable programmed allosteric control. "For this project, we used specific peptides as effectors, but any type of molecule can be used in Protein allostery occurs under the right conditions," added co-author Abbas Idris, a graduate student at the University of Washington.Illustration: Design of allosterically controlled ring assembly. (Source: paper) The researchers synthesized a number of designed proteins and then characterized the protein structure and the switching behavior caused by the binding of effector molecules. Nearly 40% of synthetic proteins designed to switch between ring assemblies composed of varying numbers of protomers are water-soluble and display the expected protomer stoichiometry.
Furthermore, the number of effectors bound to a protein follows the MWC model: all binding sites are filled, or none at all. In other words, homologous effector binding is highly cooperative and the resulting assembly does not contain a mixture of R and T protomers.
Illustration: A protein switches between assembly states by design. (Source: Nature)
Going one step further, the researchers designed proteins containing double hinges (two hinges connected by short loops) with the goal of creating structures that respond to effector binding without changing the number of protomers in the protein assembly. And the protein changes its 3D structure. Sure enough, these proteins functioned as expected, reproducing the dominant behavior of naturally occurring allosteric proteins such as hemoglobin. Finally, the researchers also designed protomers that assemble or disassemble when bound to effector molecules.
Specific de novo protein assemblies designed in the study include rings formed by the dimerization of two monomers, which upon assembly trigger light output for biosensing applications, and cage-like structures, which undergo controlled disassembly for Release the payload for drug delivery. These protein dynamics were experimentally verified in vitro by size exclusion chromatography, mass spectrometry, and electron microscopy.
Pillai emphasized that ring structures exhibit additional precise properties such as cooperativity, a phenomenon exhibited by natural systems (e.g. blood proteins, hemoglobin). In synergistic systems, the binding of one molecule enhances the binding of other molecules, creating rapid on-off reactions that are critical for precise control, such as capturing oxygen in the lungs and releasing it into tissues.
"Historically, in the laboratory, we've done a lot to control the affinity of binding a substance, such as binding it tighter and tighter. But that's not the only aspect relevant to biological systems," Pillai said. "Sometimes you want to be able to bind over a very narrow concentration range." To validate the design, the researchers characterized more than 20 protein assemblies using negative staining and cryo-electron microscopy. "This allowed us to confirm which designs formed as expected and observe how these assemblies changed their structure when effector molecules were introduced," explains Dr. Andrew Borst, head of the IPD Electron Microscopy Research Core.
Illustration: Structure characterization of sr312 and sr322 by electron microscopy. (Source: paper) 
Designed components include nanoscale containers that can be opened and closed remotely. Such systems could lead to novel drug delivery vehicles with advanced control mechanisms, including devices that sequester cell-killing drugs until they encounter tumors.
This research paves the way for the design of allosterically controlled functions beyond protein assembly and disassembly, such as regulating enzyme activity for metabolic functions and nanomachines that can convert energy into mechanical work, similar to the proteins responsible for cell movement. Actin and myosin.
“The next step is to determine whether we can form interactions with small molecules and accurately catalyze reactions, which is a more challenging frontier for the entire field,” Pillai said.
Going forward, the research team seeks to evaluate these engineered protein dynamics in a broader biological context. Future work includes installing these engineered features on cell surfaces in tissue culture, providing valuable tools for feedback control in therapeutics such as adoptive cell therapy.
Reference content:
https://www.bakerlab.org/2024/08/14/morphing-protein-assemblies-by-design/- https://www.genengnews.com/topics/artificial- intelligence/ai-designed-proteins-morph-on-demand-for-steerable-functionality/
- https://www.nature.com/articles/d41586-024-02242-7
The above is the detailed content of AI designs protein 'switches' from scratch, an amazing breakthrough in protein design, David Baker's research is published in Nature. For more information, please follow other related articles on the PHP Chinese website!
 The Hidden Dangers Of AI Internal Deployment: Governance Gaps And Catastrophic RisksApr 28, 2025 am 11:12 AM
The Hidden Dangers Of AI Internal Deployment: Governance Gaps And Catastrophic RisksApr 28, 2025 am 11:12 AMThe unchecked internal deployment of advanced AI systems poses significant risks, according to a new report from Apollo Research. This lack of oversight, prevalent among major AI firms, allows for potential catastrophic outcomes, ranging from uncont
 Building The AI PolygraphApr 28, 2025 am 11:11 AM
Building The AI PolygraphApr 28, 2025 am 11:11 AMTraditional lie detectors are outdated. Relying on the pointer connected by the wristband, a lie detector that prints out the subject's vital signs and physical reactions is not accurate in identifying lies. This is why lie detection results are not usually adopted by the court, although it has led to many innocent people being jailed. In contrast, artificial intelligence is a powerful data engine, and its working principle is to observe all aspects. This means that scientists can apply artificial intelligence to applications seeking truth through a variety of ways. One approach is to analyze the vital sign responses of the person being interrogated like a lie detector, but with a more detailed and precise comparative analysis. Another approach is to use linguistic markup to analyze what people actually say and use logic and reasoning. As the saying goes, one lie breeds another lie, and eventually
 Is AI Cleared For Takeoff In The Aerospace Industry?Apr 28, 2025 am 11:10 AM
Is AI Cleared For Takeoff In The Aerospace Industry?Apr 28, 2025 am 11:10 AMThe aerospace industry, a pioneer of innovation, is leveraging AI to tackle its most intricate challenges. Modern aviation's increasing complexity necessitates AI's automation and real-time intelligence capabilities for enhanced safety, reduced oper
 Watching Beijing's Spring Robot RaceApr 28, 2025 am 11:09 AM
Watching Beijing's Spring Robot RaceApr 28, 2025 am 11:09 AMThe rapid development of robotics has brought us a fascinating case study. The N2 robot from Noetix weighs over 40 pounds and is 3 feet tall and is said to be able to backflip. Unitree's G1 robot weighs about twice the size of the N2 and is about 4 feet tall. There are also many smaller humanoid robots participating in the competition, and there is even a robot that is driven forward by a fan. Data interpretation The half marathon attracted more than 12,000 spectators, but only 21 humanoid robots participated. Although the government pointed out that the participating robots conducted "intensive training" before the competition, not all robots completed the entire competition. Champion - Tiangong Ult developed by Beijing Humanoid Robot Innovation Center
 The Mirror Trap: AI Ethics And The Collapse Of Human ImaginationApr 28, 2025 am 11:08 AM
The Mirror Trap: AI Ethics And The Collapse Of Human ImaginationApr 28, 2025 am 11:08 AMArtificial intelligence, in its current form, isn't truly intelligent; it's adept at mimicking and refining existing data. We're not creating artificial intelligence, but rather artificial inference—machines that process information, while humans su
 New Google Leak Reveals Handy Google Photos Feature UpdateApr 28, 2025 am 11:07 AM
New Google Leak Reveals Handy Google Photos Feature UpdateApr 28, 2025 am 11:07 AMA report found that an updated interface was hidden in the code for Google Photos Android version 7.26, and each time you view a photo, a row of newly detected face thumbnails are displayed at the bottom of the screen. The new facial thumbnails are missing name tags, so I suspect you need to click on them individually to see more information about each detected person. For now, this feature provides no information other than those people that Google Photos has found in your images. This feature is not available yet, so we don't know how Google will use it accurately. Google can use thumbnails to speed up finding more photos of selected people, or may be used for other purposes, such as selecting the individual to edit. Let's wait and see. As for now
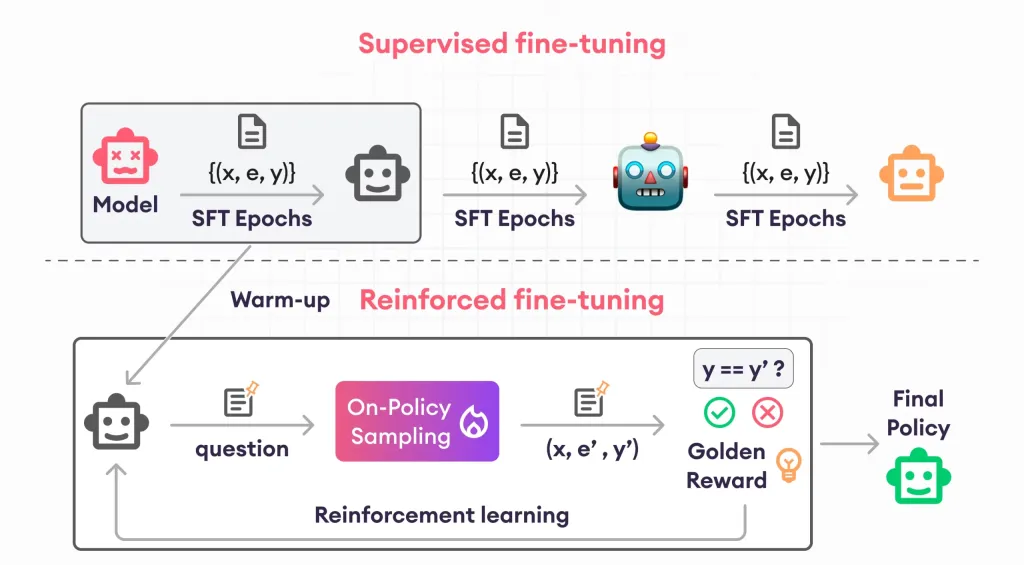 Guide to Reinforcement Finetuning - Analytics VidhyaApr 28, 2025 am 09:30 AM
Guide to Reinforcement Finetuning - Analytics VidhyaApr 28, 2025 am 09:30 AMReinforcement finetuning has shaken up AI development by teaching models to adjust based on human feedback. It blends supervised learning foundations with reward-based updates to make them safer, more accurate, and genuinely help
 Let's Dance: Structured Movement To Fine-Tune Our Human Neural NetsApr 27, 2025 am 11:09 AM
Let's Dance: Structured Movement To Fine-Tune Our Human Neural NetsApr 27, 2025 am 11:09 AMScientists have extensively studied human and simpler neural networks (like those in C. elegans) to understand their functionality. However, a crucial question arises: how do we adapt our own neural networks to work effectively alongside novel AI s


Hot AI Tools
Undresser.AI Undress
AI-powered app for creating realistic nude photos
AI Clothes Remover
Online AI tool for removing clothes from photos.

Undress AI Tool
Undress images for free

Clothoff.io
AI clothes remover
Video Face Swap
Swap faces in any video effortlessly with our completely free AI face swap tool!

Hot Article

Hot Tools

EditPlus Chinese cracked version
Small size, syntax highlighting, does not support code prompt function

Notepad++7.3.1
Easy-to-use and free code editor

Zend Studio 13.0.1
Powerful PHP integrated development environment

SublimeText3 Mac version
God-level code editing software (SublimeText3)

Atom editor mac version download
The most popular open source editor


 Research results:
Research results: 

