 Technology peripherals
Technology peripherals AI
AI The accuracy rate reaches 60.8%. Zhejiang University's chemical retrosynthesis prediction model based on Transformer was published in the Nature sub-journal
The accuracy rate reaches 60.8%. Zhejiang University's chemical retrosynthesis prediction model based on Transformer was published in the Nature sub-journal
Editor | KX
Retrosynthesis is a critical task in drug discovery and organic synthesis, and AI is increasingly used to speed up the process.
Existing AI methods have unsatisfactory performance and limited diversity. In practice, chemical reactions often cause local molecular changes, with considerable overlap between reactants and products.
Inspired by this, Hou Tingjun’s team at Zhejiang University proposed to redefine single-step retrosynthetic prediction as a molecular string editing task, and iteratively refine the target molecular string to generate precursor compounds. And an edit-based retrosynthesis model EditRetro is proposed, which can achieve high-quality and diverse predictions.
Extensive experiments show that the model achieves excellent performance on the standard benchmark data set USPTO-50 K, with a top-1 accuracy of 60.8%.
The results show that EditRetro exhibits good generalization capabilities and robustness, highlighting its potential in the field of AI-driven chemical synthesis planning.
Related research titled "Retrosynthesis prediction with an iterative string editing model" was published in "Nature Communications" on July 30.

Paper link: https://www.nature.com/articles/s41467-024-50617-1
Molecular synthesis path design is an important task in organic synthesis, which is important for biomedicine, pharmaceuticals and It is of great significance in various fields such as materials industry.
Retrosynthetic analysis is the most widely used method for developing synthetic routes. It involves using established reactions to iteratively break down molecules into simpler, easier-to-synthesize precursors.
In recent years, AI-driven retrosynthesis has facilitated the exploration of more complex molecules, greatly reducing the time and effort required to design synthetic experiments. Single-step retrosynthesis prediction is an important part of retrosynthesis planning. There are currently several deep learning-based methods with excellent results. These methods can be roughly divided into three categories: template-based methods, template-free methods, and semi-template-based methods.
Here, researchers focus on template-free retrosynthetic prediction. propose to redefine the problem as a molecular string editing task and propose EditRetro, an editing-based retrosynthetic model that can achieve high-quality and diverse predictions.

Illustration: Schematic diagram of the proposed EditRetro method based on molecular string retrosynthesis. (Source: Paper)
The core concept of this research is to generate reactant strings through an iterative editing process using Levenshtein operations. The approach draws inspiration from recent advances in edit-based sequence generation models. Specifically, operations from EDITOR, an editing-based Transformer designed for neural machine translation, are employed.
EditRetro Overview
The EditRetro model contains three editing operations, namely sequence relocation, placeholder insertion and marker insertion, to generate reactant strings. It is implemented by a Transformer model, which consists of an encoder and three decoders, both consisting of stacked Transformer blocks.
- Relocation decoder: Relocation operations include basic token editing operations such as retain, delete, and reorder. It can be compared to the process of identifying reaction centers, including reordering and deleting atoms or groups to obtain synthons.
- Placeholder decoder: The placeholder insertion strategy (classifier) predicts the number of placeholders to insert between adjacent tokens. It plays a crucial role in determining the structure of reactants, similar to identifying the positions of added atoms or groups in intermediate synthons obtained from the sequence repositioning stage.
- Token decoder: token insertion strategy (classifier), responsible for generating candidate tokens for each placeholder. This is crucial to determine the actual reactants that can be used to synthesize the target product. This process can be viewed as a similar process done by synthons, combined with placeholder insertion operations.
EditRetro model improves generation efficiency through its non-autoregressive decoder. Although incorporating additional decoders to iteratively predict editing operations, EditRetro performs editing operations in parallel within each decoder (i.e., non-autoregressive generation).
When given a target molecule, the encoder takes its string as input and generates the corresponding hidden representation, which is then used as input to the decoder’s cross-attention module. Similarly, the decoder also takes the product string as input on the first iteration. During each decoding iteration, the three decoders are executed sequentially.
Better than baseline, generate accurate reactants
연구원들은 공개 벤치마크 데이터 세트 USPTO-50K 및 USPTO-FULL에서 제안된 방법을 평가했습니다. 광범위한 실험 결과에 따르면 이 방법은 최첨단 시퀀스 기반 방법인 R-SMILES 및 그래프 편집 기반 방법인 Graph2Edits를 포함하여 예측 정확도 측면에서 다른 기준보다 성능이 뛰어납니다.

EditRetro 벤치마크 역합성 데이터 세트 USPTO-50K에 대한 광범위한 실험에서 EditRetro는 60.8%의 상위 1개 완전 일치 정확도로 탁월한 성능을 달성하는 것으로 나타났습니다.

또한 대규모 USPTO-FULL 데이터 세트에서 상위 1위의 정확한 일치 정확도가 52.2%에 달해 더욱 다양하고 까다로운 화학 반응에서도 그 효과가 입증되었습니다.

EditRetro는 또한 RoundTrip 및 MaxFrag 정확도 측면에서 기본 방법보다 더 나은 성능을 보여줍니다. 이는 EditRetro가 화학적 규칙을 효과적으로 학습할 수 있음을 보여줍니다.
또한 EditRetro는 잘 설계된 추론 모듈을 통해 다양한 예측을 제공합니다. 이 모듈은 재배치 샘플링과 시퀀스 확대를 결합하여 다양하고 변화하는 예측을 생성하는 데 도움을 줍니다. 재배치 샘플링 샘플 재배치 작업에 대한 예측을 통해 고유한 응답 사이트를 식별할 수 있습니다. 서열 향상은 다양한 제품 변형에서 반응물까지 다양한 편집 경로를 생성하여 예측 정확도와 다양성을 높입니다. 이 두 가지 전략은 함께 작동하여 예측의 정확성과 다양성을 높입니다.
추가 실험을 통해 키랄, 고리 열림 및 고리 형성 반응을 포함한 좀 더 복잡한 반응에서 EditRetro의 우수성이 입증되었습니다. 결과는 이러한 까다로운 시나리오에서 EditRetro의 우수성을 확인하고 다양한 유형의 화학적 변형을 처리할 수 있는 능력을 보여줍니다.
다단계 합성 계획의 실용성
특히 4가지 다단계 역합성 계획 시나리오에서 EditRetro를 성공적으로 적용한 것은 그 실용성을 입증합니다.
합성 계획에서 EditRetro의 유용성을 평가하기 위해 순차적 역합성 예측을 통해 완전한 화학 경로를 설계했습니다. 연구진은 평가를 위해 중요한 약학적 가치를 지닌 4가지 표적 화합물, 즉 페북소스타트(febuxostat), 오시머티닙(osimertinib), GPX4의 알로스테릭 활성화제, DDR1 키나제 억제제 INS015_037을 선택했습니다.

그림: EditRetro의 다단계 역합성 예측. (출처: 논문)
네 가지 사례 모두 문헌에 보고된 것과 매우 일치하는 역합성 경로를 생성했으며 대부분의 예측이 상위 2위에 올랐습니다. 고려된 16개의 개별 단계 중 10개의 예측 정확도는 1이었습니다. 이러한 결과는 실제 역합성 예측에서 EditRetro의 실질적인 잠재력을 보여줍니다.
이 방법은 귀중한 통찰력을 제공하고 효율적인 합성 경로 설계를 촉진함으로써 역합성 계획 분야에서 실용적인 응용 프로그램을 찾을 것으로 예상됩니다.
The above is the detailed content of The accuracy rate reaches 60.8%. Zhejiang University's chemical retrosynthesis prediction model based on Transformer was published in the Nature sub-journal. For more information, please follow other related articles on the PHP Chinese website!
 Tesla's Robovan Was The Hidden Gem In 2024's Robotaxi TeaserApr 22, 2025 am 11:48 AM
Tesla's Robovan Was The Hidden Gem In 2024's Robotaxi TeaserApr 22, 2025 am 11:48 AMSince 2008, I've championed the shared-ride van—initially dubbed the "robotjitney," later the "vansit"—as the future of urban transportation. I foresee these vehicles as the 21st century's next-generation transit solution, surpas
 Sam's Club Bets On AI To Eliminate Receipt Checks And Enhance RetailApr 22, 2025 am 11:29 AM
Sam's Club Bets On AI To Eliminate Receipt Checks And Enhance RetailApr 22, 2025 am 11:29 AMRevolutionizing the Checkout Experience Sam's Club's innovative "Just Go" system builds on its existing AI-powered "Scan & Go" technology, allowing members to scan purchases via the Sam's Club app during their shopping trip.
 Nvidia's AI Omniverse Expands At GTC 2025Apr 22, 2025 am 11:28 AM
Nvidia's AI Omniverse Expands At GTC 2025Apr 22, 2025 am 11:28 AMNvidia's Enhanced Predictability and New Product Lineup at GTC 2025 Nvidia, a key player in AI infrastructure, is focusing on increased predictability for its clients. This involves consistent product delivery, meeting performance expectations, and
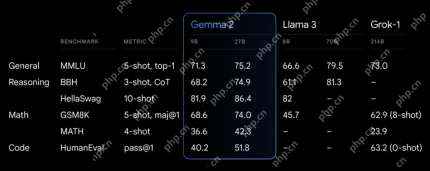 Exploring the Capabilities of Google's Gemma 2 ModelsApr 22, 2025 am 11:26 AM
Exploring the Capabilities of Google's Gemma 2 ModelsApr 22, 2025 am 11:26 AMGoogle's Gemma 2: A Powerful, Efficient Language Model Google's Gemma family of language models, celebrated for efficiency and performance, has expanded with the arrival of Gemma 2. This latest release comprises two models: a 27-billion parameter ver
 The Next Wave of GenAI: Perspectives with Dr. Kirk Borne - Analytics VidhyaApr 22, 2025 am 11:21 AM
The Next Wave of GenAI: Perspectives with Dr. Kirk Borne - Analytics VidhyaApr 22, 2025 am 11:21 AMThis Leading with Data episode features Dr. Kirk Borne, a leading data scientist, astrophysicist, and TEDx speaker. A renowned expert in big data, AI, and machine learning, Dr. Borne offers invaluable insights into the current state and future traje
 AI For Runners And Athletes: We're Making Excellent ProgressApr 22, 2025 am 11:12 AM
AI For Runners And Athletes: We're Making Excellent ProgressApr 22, 2025 am 11:12 AMThere were some very insightful perspectives in this speech—background information about engineering that showed us why artificial intelligence is so good at supporting people’s physical exercise. I will outline a core idea from each contributor’s perspective to demonstrate three design aspects that are an important part of our exploration of the application of artificial intelligence in sports. Edge devices and raw personal data This idea about artificial intelligence actually contains two components—one related to where we place large language models and the other is related to the differences between our human language and the language that our vital signs “express” when measured in real time. Alexander Amini knows a lot about running and tennis, but he still
 Jamie Engstrom On Technology, Talent And Transformation At CaterpillarApr 22, 2025 am 11:10 AM
Jamie Engstrom On Technology, Talent And Transformation At CaterpillarApr 22, 2025 am 11:10 AMCaterpillar's Chief Information Officer and Senior Vice President of IT, Jamie Engstrom, leads a global team of over 2,200 IT professionals across 28 countries. With 26 years at Caterpillar, including four and a half years in her current role, Engst
 New Google Photos Update Makes Any Photo Pop With Ultra HDR QualityApr 22, 2025 am 11:09 AM
New Google Photos Update Makes Any Photo Pop With Ultra HDR QualityApr 22, 2025 am 11:09 AMGoogle Photos' New Ultra HDR Tool: A Quick Guide Enhance your photos with Google Photos' new Ultra HDR tool, transforming standard images into vibrant, high-dynamic-range masterpieces. Ideal for social media, this tool boosts the impact of any photo,


Hot AI Tools

Undresser.AI Undress
AI-powered app for creating realistic nude photos

AI Clothes Remover
Online AI tool for removing clothes from photos.

Undress AI Tool
Undress images for free

Clothoff.io
AI clothes remover

Video Face Swap
Swap faces in any video effortlessly with our completely free AI face swap tool!

Hot Article

Hot Tools

PhpStorm Mac version
The latest (2018.2.1) professional PHP integrated development tool
ZendStudio 13.5.1 Mac
Powerful PHP integrated development environment

WebStorm Mac version
Useful JavaScript development tools

Safe Exam Browser
Safe Exam Browser is a secure browser environment for taking online exams securely. This software turns any computer into a secure workstation. It controls access to any utility and prevents students from using unauthorized resources.

Notepad++7.3.1
Easy-to-use and free code editor




