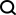Home >Technology peripherals >AI >FDA approves an AI medical image that can identify cerebral hemorrhage in one minute
FDA approves an AI medical image that can identify cerebral hemorrhage in one minute
- PHPzforward
- 2023-11-18 16:33:101319browse
·Rapid SDH has a sensitivity of 92.4% and a specificity of 98.7% in identifying possible cerebral hemorrhage.

Fast SDH. Picture taken from RapidAI official website
On November 9, 2023 local time, RapidAI, an American company specializing in artificial intelligence medical image analysis, announced that the U.S. Food and Drug Administration (FDA) approved its Rapid SDH for use in U.S. hospitals. Rapid SDH is a cutting-edge tool for detecting and reporting conditions such as suspected acute and chronic subdural hematomas on non-contrast CT scans.
Rapid SDH is powered by AI (artificial intelligence) trained with past patient data to discover potential indicators of acute and chronic subdural hematoma, allowing clinicians to diagnose patients with traumatic brain injury or hemorrhagic stroke Patients make more timely, informed referral and treatment decisions.
According to data from the RapidAI official website, Rapid SDH has a sensitivity of 92.4% and a specificity of 98.7% in detecting potential cerebral hemorrhage
It is reported that subdural hematoma is a kind of intracranial hemorrhage, which refers to the accumulation of hematoma between the dura mater and the arachnoid mater. It is more common in patients with craniocerebral trauma. It is often caused by car accidents, falling from a height, or falling to the head. The head was hit.
The exact incidence of subdural hematoma is unknown, but one study showed that approximately 11% of patients with mild to moderate head injuries requiring hospitalization developed acute subdural hematoma; Approximately 20% of patients with traumatic brain injury develop acute subdural hematoma. The average age of patients with subdural hematoma caused by head injury is 30 to 50 years old, and most of them are men
The incidence of chronic subdural hematoma is 1.7-20.6/100,000 per year, and it mainly occurs in the elderly with a history of minor head trauma. This risk appears to be gradually increasing, possibly because of the aging population and increased use of antiplatelet and anticoagulant drugs. The number of patients with subdural hematoma in the United States is expected to increase by nearly 80% by 2040, with mortality currently estimated to be 40-60%
Amit Phadnis, technical director at RapidAI, said: “The FDA’s approval of Rapid SDH significantly enhances our expanding range of bleeding and trauma care solutions at a critical time when patient volumes are growing rapidly, clinician shortages are increasing, and potential treatment options are advancing. .”
RapidAI said the Rapid SDH is capable of performing brain CT scans and transmitting the results to doctors within one minute. The results are available via the RapidAI mobile app, email, and the hospital's existing picture archiving and communication system (PACS), allowing each member of the trauma team to view and assess the results themselves and review the patient's case remotely if needed. Classification
Rapid SDH can be used with RapidAI’s other AI-powered modules, Rapid ICH and Rapid Hyperdensity, which are trained to capture signs of intracranial hemorrhage and high-density areas of the brain. This three-in-one solution is designed to help hospitals speed up the process of evaluating potential cases of traumatic brain injury and hemorrhagic stroke and begin treating diagnosed patients as quickly as possible.
RapidAI is the world’s leading company using artificial intelligence to fight life-threatening vascular and neurovascular diseases. In June 2020, Rapid ASPECTS received separate FDA clearance for computer-aided diagnostic software (CADx category), making it the first neuroimaging solution in history to be cleared in the CADx category
According to a RapidAI press release, Rapid ASPECTS uses proven machine learning algorithms to automatically identify ASPECTS regions of the brain to generate a standardized Alberta Stroke Program Early CT Score (ASPECTS score) to indicate brain infarction on non-contrast CT scans Early signs help doctors identify areas of irreversible brain damage.
RapidAI co-founder Greg Albers, professor of neurology at Stanford University and director of the Stanford Stroke Center, said: "Rapid ASPECTS represents a step forward in artificial intelligence-driven stroke imaging, and the FDA's breakthrough CADx clearance makes it unique. .
Since then, RapidAI has continued to research new technologies and continuously trained and improved the performance of its machine learning algorithms by gathering rich data from more than 1,600 hospitals and 50 countries
RapidAI CEO Karim Karti said: “Hospitals are increasingly looking to RapidAI solutions to address their critical needs, including helping care teams work more effectively and supporting clinical decision-making.”
The American company Viz.AI received FDA approval as early as 2022 and became a pioneer focused on building an AI-driven cardiovascular and cerebrovascular diagnosis and in-hospital diagnosis and treatment standardization platform. Their AI tool Viz SDH was applied to the detection of acute and chronic subdural hematomas in brain CT images. A multicenter study involving more than 500 participants showed Viz SDH was 94% sensitive and 92% specific in detecting subdural hemorrhage
The development of AI and medical technology has brought various possibilities to the current and future medical imaging fields. On May 25, 2023, the New England Journal of Medicine (NEJM) published an article stating that companies in more than 20 countries have developed commercial AI algorithms, and some hospitals and other point-of-care testing centers have successfully applied AI products. Most radiologists and residents expect that the imaging profession will undergo significant changes over the next decade and believe that AI should have a "co-pilot" role, serving as a second reader and improving workflow tasks.
The original meaning will not be changed when rewriting the content. The language that needs to be rewritten is Chinese, and the original sentence does not need to appear
The content that needs to be rewritten is: 1. https://www.fiercebiotech.com/medtech/rapidai-earns-fda-nod-subdural-hematoma-spotting-ai
2. https://mp.weixin.qq.com/s/4viEjuCE46EpxPzLwjY5ag
The above is the detailed content of FDA approves an AI medical image that can identify cerebral hemorrhage in one minute. For more information, please follow other related articles on the PHP Chinese website!
Related articles
See more- Plug in the electrodes and Musk will cut your brain! Neuralink clinical trial approved by FDA, brain-computer interface project reaches new milestone
- Heavy! Musk's brain-computer interface company has been approved by the FDA and is about to conduct human trials [with forecast of the future of brain-computer interface]
- Novarad HoloLens AR surgical navigation system VisAR receives approval from Indonesian FDA