 Technology peripherals
Technology peripherals AI
AI Musk's brain-computer interface company Neuralink is approved to launch the first human clinical trial | Titanium News
Musk's brain-computer interface company Neuralink is approved to launch the first human clinical trial | Titanium NewsMusk's brain-computer interface company Neuralink is approved to launch the first human clinical trial | Titanium News

(Photo source: File Photo)
Titanium Media App News on May 26, This morning, Beijing time, Musk’s brain-computer interface company Neuralink stated on social platforms that the company has obtained approval from the U.S. Food and Drug Administration (FDA) With approval, the first human clinical study of brain implants will be launched, but clinical trial recruitment has not yet begun.
"This is the result of incredible work by the Neuralink team working closely with the FDA and represents an important first step in how our technology will one day help many people," Neuralink said in a tweet.

This means that Neuralink has become the second brain-computer interface company to be approved by the US FDA to enter the first human clinical trial after Synchron. It also indicates that Neuralink is moving towards the commercialization of the "whole brain interface" that Musk hopes to achieve. goal is one step closer.
In fact, Neuralink’s FDA approval process has been full of twists and turns.
Neuralink was founded in 2016. It is committed to the research of brain-computer interface technology. It is developing a brain implant that uses invasive brain-computer interface technology to implant tiny electrodes in the brain and use electric current to Computers interact with brain cells in hopes of helping paralyzed people walk again and curing other neurological diseases.
Musk has predicted for at least the fourth time since 2019 that Neuralink will soon receive FDA approval for human trials. The company's application was rejected in early 2022 because it failed to address safety concerns about the experimental implants. Musk announced in December that Neuralink would begin human clinical trials within the next six months.
- In September 2019, Musk announced that Neuralink had developed the "brain-machine neural mesh" device N1, which attaches more than 3,000 electrodes to flexible filaments, which may enable the brain to control computers. Since then, research on brain-computer interfaces has been pushed into the public eye and has become a current hot topic.
- In August 2020, Musk once again released the latest research results of Neuralink and realized the experiment of implanting a brain-computer interface in pigs: using a coin-sized implanted chip device Link V0.9 and a surgical robot V2, This "coin" device is implanted into the cerebral cortex part of the human skull to complete the implantation of the brain-computer interface. The machine obtains the electrode signals inside the brain and transmits them to mobile phones, computers and other devices, completing data transmission between brain and computer in real time.
- In April 2021, Neuralink went a step further and conducted a brain-computer interface experiment on a macaque named "Pager", enabling a monkey to use only its brain without a game joystick. Use your thoughts to control a computer and play table tennis.
- In December 2022, Musk announced that through multiple device/hardware upgrades, Neuralink has enabled a monkey named Sake to use his brain to control a virtual keyboard to type, completing fast "thought typing". And it is expected to start human clinical trials within the next 6 months.
According to Reuters, the U.S. government has begun investigating Neuralink after the FDA approved it. Earlier this month, U.S. lawmakers urged the U.S. Department of Agriculture and other departments to investigate whether oversight of Neuralink’s animal testing led to innocent animal deaths and rushed experimental results. In December, the U.S. Department of Agriculture’s inspector general began investigating whether Neuralink violated animal welfare laws at the request of federal prosecutors.
Neuralink said in a tweet this morning that the company is not yet open for clinical trials.
Musk once revealed that the next generation of Neuralink products, in addition to the two major products of brain-computer interface chips and electrodes, and surgical robots, the team is developing implants that enter the spinal cord and may restore the movement ability of paralyzed patients, and Vision-restoring eye implants implanted in the visual cortex of two monkeys. Neuralink's goal is to bring light back to those who have lived in darkness for years.
In the seven years since its establishment, Neuralink has completed a total of more than 160 million US dollars (approximately 1.133 billion yuan) in financing, most of which came from Musk himself. Data disclosed by PitchBook in 2020 shows that Neuralink's valuation exceeds US$500 million. Other news shows that as of January this year, Neuralink's expected valuation is as high as US$5.5 billion. (This article was first published on Titanium Media App, author | Lin Zhijia)
The above is the detailed content of Musk's brain-computer interface company Neuralink is approved to launch the first human clinical trial | Titanium News. For more information, please follow other related articles on the PHP Chinese website!
 Personal Hacking Will Be A Pretty Fierce BearMay 11, 2025 am 11:09 AM
Personal Hacking Will Be A Pretty Fierce BearMay 11, 2025 am 11:09 AMCyberattacks are evolving. Gone are the days of generic phishing emails. The future of cybercrime is hyper-personalized, leveraging readily available online data and AI to craft highly targeted attacks. Imagine a scammer who knows your job, your f
 Pope Leo XIV Reveals How AI Influenced His Name ChoiceMay 11, 2025 am 11:07 AM
Pope Leo XIV Reveals How AI Influenced His Name ChoiceMay 11, 2025 am 11:07 AMIn his inaugural address to the College of Cardinals, Chicago-born Robert Francis Prevost, the newly elected Pope Leo XIV, discussed the influence of his namesake, Pope Leo XIII, whose papacy (1878-1903) coincided with the dawn of the automobile and
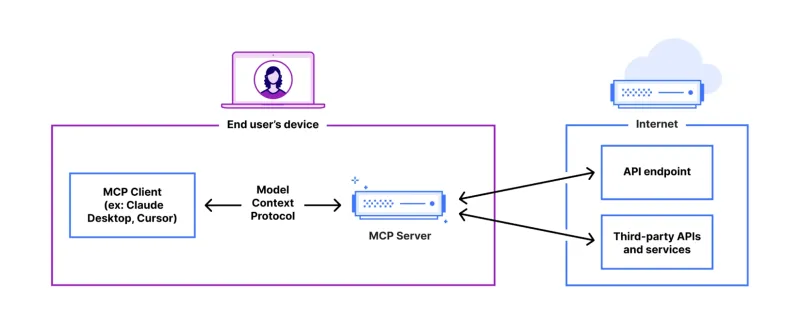 FastAPI-MCP Tutorial for Beginners and Experts - Analytics VidhyaMay 11, 2025 am 10:56 AM
FastAPI-MCP Tutorial for Beginners and Experts - Analytics VidhyaMay 11, 2025 am 10:56 AMThis tutorial demonstrates how to integrate your Large Language Model (LLM) with external tools using the Model Context Protocol (MCP) and FastAPI. We'll build a simple web application using FastAPI and convert it into an MCP server, enabling your L
 Dia-1.6B TTS : Best Text-to-Dialogue Generation Model - Analytics VidhyaMay 11, 2025 am 10:27 AM
Dia-1.6B TTS : Best Text-to-Dialogue Generation Model - Analytics VidhyaMay 11, 2025 am 10:27 AMExplore Dia-1.6B: A groundbreaking text-to-speech model developed by two undergraduates with zero funding! This 1.6 billion parameter model generates remarkably realistic speech, including nonverbal cues like laughter and sneezes. This article guide
 3 Ways AI Can Make Mentorship More Meaningful Than EverMay 10, 2025 am 11:17 AM
3 Ways AI Can Make Mentorship More Meaningful Than EverMay 10, 2025 am 11:17 AMI wholeheartedly agree. My success is inextricably linked to the guidance of my mentors. Their insights, particularly regarding business management, formed the bedrock of my beliefs and practices. This experience underscores my commitment to mentor
 AI Unearths New Potential In The Mining IndustryMay 10, 2025 am 11:16 AM
AI Unearths New Potential In The Mining IndustryMay 10, 2025 am 11:16 AMAI Enhanced Mining Equipment The mining operation environment is harsh and dangerous. Artificial intelligence systems help improve overall efficiency and security by removing humans from the most dangerous environments and enhancing human capabilities. Artificial intelligence is increasingly used to power autonomous trucks, drills and loaders used in mining operations. These AI-powered vehicles can operate accurately in hazardous environments, thereby increasing safety and productivity. Some companies have developed autonomous mining vehicles for large-scale mining operations. Equipment operating in challenging environments requires ongoing maintenance. However, maintenance can keep critical devices offline and consume resources. More precise maintenance means increased uptime for expensive and necessary equipment and significant cost savings. AI-driven
 Why AI Agents Will Trigger The Biggest Workplace Revolution In 25 YearsMay 10, 2025 am 11:15 AM
Why AI Agents Will Trigger The Biggest Workplace Revolution In 25 YearsMay 10, 2025 am 11:15 AMMarc Benioff, Salesforce CEO, predicts a monumental workplace revolution driven by AI agents, a transformation already underway within Salesforce and its client base. He envisions a shift from traditional markets to a vastly larger market focused on
 AI HR Is Going To Rock Our Worlds As AI Adoption SoarsMay 10, 2025 am 11:14 AM
AI HR Is Going To Rock Our Worlds As AI Adoption SoarsMay 10, 2025 am 11:14 AMThe Rise of AI in HR: Navigating a Workforce with Robot Colleagues The integration of AI into human resources (HR) is no longer a futuristic concept; it's rapidly becoming the new reality. This shift impacts both HR professionals and employees, dem


Hot AI Tools

Undresser.AI Undress
AI-powered app for creating realistic nude photos

AI Clothes Remover
Online AI tool for removing clothes from photos.

Undress AI Tool
Undress images for free

Clothoff.io
AI clothes remover

Video Face Swap
Swap faces in any video effortlessly with our completely free AI face swap tool!

Hot Article

Hot Tools

Notepad++7.3.1
Easy-to-use and free code editor

SublimeText3 Chinese version
Chinese version, very easy to use

Zend Studio 13.0.1
Powerful PHP integrated development environment

SublimeText3 Linux new version
SublimeText3 Linux latest version

WebStorm Mac version
Useful JavaScript development tools





