 Technology peripherals
Technology peripherals AI
AI 'Resurrection' of ancient biological molecules, AI solves antibiotic resistance, two papers published by Fudan University and Penn University collaborative teams were published in Cell and Nature sub-journals
'Resurrection' of ancient biological molecules, AI solves antibiotic resistance, two papers published by Fudan University and Penn University collaborative teams were published in Cell and Nature sub-journals
編輯| 蘿蔔皮
抗生素抗藥性感染每年在全球造成約127 萬人死亡,預計到2050 年,如果沒有特效的新藥,每年死亡人數將達到1000 萬人,因此需要採取緊急措施來應對抗生素抗藥性。
賓州大學的校長助理教授(Presidential Assistant Professor) Cesar de la Fuente 說:「即使感覺身體好些了,也要確保完成抗生素療程,這是許多人聽過,但經常忽視的醫學口頭禪。 「近幾十年來,這導致了抗藥性細菌的增加,全球健康危機日益嚴重,每年造成約495 萬人死亡,甚至可能使普通感染也致命。」
##De la Fuente 和復旦大學、賓州大學的研究人員組成的跨領域研究團隊,一直致力於研究應對抗生素抗藥性問題。 在最新的研究中,他們開發了一種人工智慧工具來挖掘龐大且基本上未開發的生物數據——超過1000萬個現代和已滅絕生物的分子——以發現新的抗生素候選藥物。 研究以「Deep-learning-enabled antibiotic discovery through molecular de-extinction
」為題,於2024 年6 月11 日發佈於《Nature Biomedical Engineering》。
論文連結: https://www.nature.com/articles/s41551-024-01201-x
https://www.nature.com/articles/s41551-024-01201-x
「分子復活」技術
De la Fuente 團隊開發了所謂的「分子復活」技術,即復活已經滅絕的具有潛在治療作用的古代分子,並因此在古代生物的基因組中發現了治療分子。他們推測,他們發現的許多分子可能在整個進化過程中為宿主的免疫發揮作用。 研究以「Discovery of antimicrobial peptides in the global microbiome with machine learning」為題,於 2024 年 6 月 5 日發佈在《Cell
》。論文連結:https://doi.org/10.1016/j.cell.2024.05.013
#研究者在《 Cell
Cell

A total of 79 peptides were active, 63 of which targeted pathogens. These active AMPs exhibit antimicrobial activity by disrupting bacterial membranes. In total, this approach identified nearly one million prokaryotic AMP sequences, an open source for antibiotic discovery.
antibiotic peptide de-extinction
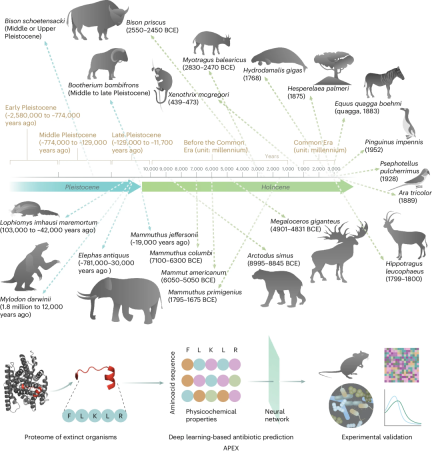
In the study in "Nature Biomedical Engineering, researchers show that deep learning can be used to mine the proteomes of all available extinct organisms to discover antibiotic peptides.
De la Fuente’s team trained a combination of deep learning models consisting of peptide sequence encoders and neural networks, called Antibiotic Peptide De-Extinction (APEX), to predict antimicrobial activity and used it to mine 10,311,899 peptides .
Marcelo Der Torossian Torres, a postdoctoral researcher in De la Fuente's lab, said that when the team built APEX, they first created a "highly standardized data set to train it, which was missing in the literature... This is surprising because there are so many data sets and researchers will use multiple data sets, assuming that all samples are collected in a very systematic and consistent way, which is not always the case."
APEX did also utilize "probably the largest data set of its kind" as a control for the experiment, he said. This allows researchers to determine how their models perform relative to existing knowledge and validate the uniqueness and validity of antibiotic sequences discovered by APEX.
"Only with high-quality data sets can artificial intelligence succeed in a complex and messy field like biology." De la Fuente said, "We realized this many years ago and have been working hard. Create a data set that can be used to train our algorithm."
APEX uses a combination of recurrent neural networks and attention networks to perform two key tasks, namely identifying encryption," said Fangping Wan, a postdoctoral researcher in De la Fuente's lab. Peptides, fragments within proteins that have antimicrobial properties.
"Recurrent neural networks are very good at processing sequences, such as proteins, because they can process input independent and ordered data." Wan said, "And attention networks can improve the network's localization of proteins that may be related to antibacterial activity. The models predicted 37,176 sequences with broad-spectrum antibacterial activity, 11,035 of which were not found in extant organisms.
Synthesis and Application ValidationThey also synthesized 69 peptides and experimentally confirmed their activity against bacterial pathogens. Most peptides kill bacteria by depolarizing their cytoplasmic membrane, in contrast to known antimicrobial peptides, which tend to target the outer membrane.
It is worth noting that some of the lead compounds (including mammothin-2 from mammoths, pixel-2 from straight-tusked elephants, hydrogenated damin-1 from ancient manatees, from giant trees Lazy carnosine-2 and macrocerocin-1 from the extinct giant elk) showed anti-infectious activity in mice with skin abscesses or thigh infections.
This is a crucial step as it brings these drug candidates closer to potential clinical trials and eventual therapeutic use.
In addition, most of the ancient peptides have a novel mechanism of action by depolarizing bacterial cell membranes, a unique targeting approach that suggests a new paradigm for infectious disease control.
Taken together, the computational work performed by De la Fuente’s lab over the past five years has significantly accelerated the ability to discover new antibiotics. What used to take years of hard work using traditional methods can now be done in just a few hours using AI.
Related reports:
https://phys.org/news/2024-06-ai-antibiotic-resistance.htmlThe above is the detailed content of 'Resurrection' of ancient biological molecules, AI solves antibiotic resistance, two papers published by Fudan University and Penn University collaborative teams were published in Cell and Nature sub-journals. For more information, please follow other related articles on the PHP Chinese website!
 2023年机器学习的十大概念和技术Apr 04, 2023 pm 12:30 PM
2023年机器学习的十大概念和技术Apr 04, 2023 pm 12:30 PM机器学习是一个不断发展的学科,一直在创造新的想法和技术。本文罗列了2023年机器学习的十大概念和技术。 本文罗列了2023年机器学习的十大概念和技术。2023年机器学习的十大概念和技术是一个教计算机从数据中学习的过程,无需明确的编程。机器学习是一个不断发展的学科,一直在创造新的想法和技术。为了保持领先,数据科学家应该关注其中一些网站,以跟上最新的发展。这将有助于了解机器学习中的技术如何在实践中使用,并为自己的业务或工作领域中的可能应用提供想法。2023年机器学习的十大概念和技术:1. 深度神经网
 超参数优化比较之网格搜索、随机搜索和贝叶斯优化Apr 04, 2023 pm 12:05 PM
超参数优化比较之网格搜索、随机搜索和贝叶斯优化Apr 04, 2023 pm 12:05 PM本文将详细介绍用来提高机器学习效果的最常见的超参数优化方法。 译者 | 朱先忠审校 | 孙淑娟简介通常,在尝试改进机器学习模型时,人们首先想到的解决方案是添加更多的训练数据。额外的数据通常是有帮助(在某些情况下除外)的,但生成高质量的数据可能非常昂贵。通过使用现有数据获得最佳模型性能,超参数优化可以节省我们的时间和资源。顾名思义,超参数优化是为机器学习模型确定最佳超参数组合以满足优化函数(即,给定研究中的数据集,最大化模型的性能)的过程。换句话说,每个模型都会提供多个有关选项的调整“按钮
 人工智能自动获取知识和技能,实现自我完善的过程是什么Aug 24, 2022 am 11:57 AM
人工智能自动获取知识和技能,实现自我完善的过程是什么Aug 24, 2022 am 11:57 AM实现自我完善的过程是“机器学习”。机器学习是人工智能核心,是使计算机具有智能的根本途径;它使计算机能模拟人的学习行为,自动地通过学习来获取知识和技能,不断改善性能,实现自我完善。机器学习主要研究三方面问题:1、学习机理,人类获取知识、技能和抽象概念的天赋能力;2、学习方法,对生物学习机理进行简化的基础上,用计算的方法进行再现;3、学习系统,能够在一定程度上实现机器学习的系统。
 得益于OpenAI技术,微软必应的搜索流量超过谷歌Mar 31, 2023 pm 10:38 PM
得益于OpenAI技术,微软必应的搜索流量超过谷歌Mar 31, 2023 pm 10:38 PM截至3月20日的数据显示,自微软2月7日推出其人工智能版本以来,必应搜索引擎的页面访问量增加了15.8%,而Alphabet旗下的谷歌搜索引擎则下降了近1%。 3月23日消息,外媒报道称,分析公司Similarweb的数据显示,在整合了OpenAI的技术后,微软旗下的必应在页面访问量方面实现了更多的增长。截至3月20日的数据显示,自微软2月7日推出其人工智能版本以来,必应搜索引擎的页面访问量增加了15.8%,而Alphabet旗下的谷歌搜索引擎则下降了近1%。这些数据是微软在与谷歌争夺生
 荣耀的人工智能助手叫什么名字Sep 06, 2022 pm 03:31 PM
荣耀的人工智能助手叫什么名字Sep 06, 2022 pm 03:31 PM荣耀的人工智能助手叫“YOYO”,也即悠悠;YOYO除了能够实现语音操控等基本功能之外,还拥有智慧视觉、智慧识屏、情景智能、智慧搜索等功能,可以在系统设置页面中的智慧助手里进行相关的设置。
 30行Python代码就可以调用ChatGPT API总结论文的主要内容Apr 04, 2023 pm 12:05 PM
30行Python代码就可以调用ChatGPT API总结论文的主要内容Apr 04, 2023 pm 12:05 PM阅读论文可以说是我们的日常工作之一,论文的数量太多,我们如何快速阅读归纳呢?自从ChatGPT出现以后,有很多阅读论文的服务可以使用。其实使用ChatGPT API非常简单,我们只用30行python代码就可以在本地搭建一个自己的应用。 阅读论文可以说是我们的日常工作之一,论文的数量太多,我们如何快速阅读归纳呢?自从ChatGPT出现以后,有很多阅读论文的服务可以使用。其实使用ChatGPT API非常简单,我们只用30行python代码就可以在本地搭建一个自己的应用。使用 Python 和 C
 人工智能在教育领域的应用主要有哪些Dec 14, 2020 pm 05:08 PM
人工智能在教育领域的应用主要有哪些Dec 14, 2020 pm 05:08 PM人工智能在教育领域的应用主要有个性化学习、虚拟导师、教育机器人和场景式教育。人工智能在教育领域的应用目前还处于早期探索阶段,但是潜力却是巨大的。
 人工智能在生活中的应用有哪些Jul 20, 2022 pm 04:47 PM
人工智能在生活中的应用有哪些Jul 20, 2022 pm 04:47 PM人工智能在生活中的应用有:1、虚拟个人助理,使用者可通过声控、文字输入的方式,来完成一些日常生活的小事;2、语音评测,利用云计算技术,将自动口语评测服务放在云端,并开放API接口供客户远程使用;3、无人汽车,主要依靠车内的以计算机系统为主的智能驾驶仪来实现无人驾驶的目标;4、天气预测,通过手机GPRS系统,定位到用户所处的位置,在利用算法,对覆盖全国的雷达图进行数据分析并预测。


Hot AI Tools

Undresser.AI Undress
AI-powered app for creating realistic nude photos

AI Clothes Remover
Online AI tool for removing clothes from photos.

Undress AI Tool
Undress images for free

Clothoff.io
AI clothes remover

AI Hentai Generator
Generate AI Hentai for free.

Hot Article

Hot Tools

SublimeText3 English version
Recommended: Win version, supports code prompts!

Zend Studio 13.0.1
Powerful PHP integrated development environment

Atom editor mac version download
The most popular open source editor

MinGW - Minimalist GNU for Windows
This project is in the process of being migrated to osdn.net/projects/mingw, you can continue to follow us there. MinGW: A native Windows port of the GNU Compiler Collection (GCC), freely distributable import libraries and header files for building native Windows applications; includes extensions to the MSVC runtime to support C99 functionality. All MinGW software can run on 64-bit Windows platforms.

Dreamweaver Mac version
Visual web development tools




